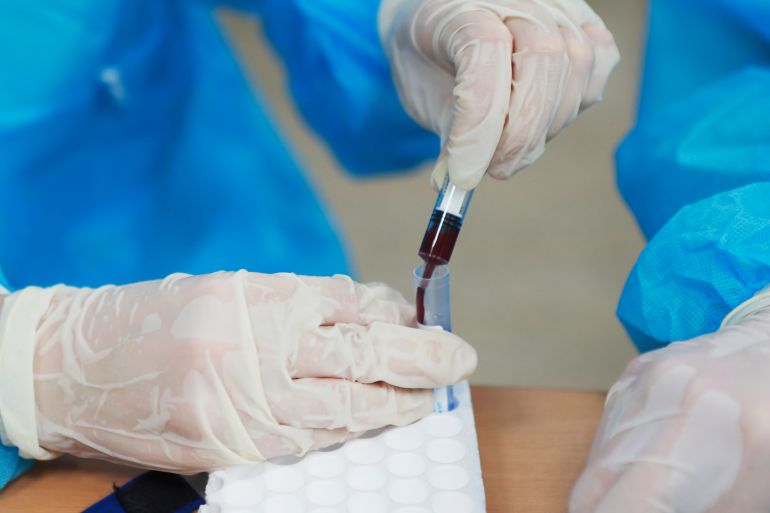
Today, scientists are exploring the production of artificial blood to address the shortage of blood and the need for safe blood transfusions. According to research by the World Health Organization, millions of people worldwide die each year due to lack of access to blood. In the first clinical trial in 2022, laboratory cultured blood was used in humans, especially for patients with rare blood types. Scientists are also committed to developing synthetic blood to support emergency medical care, surgical procedures, and blood transfusions.
Firstly, artificial blood is a broad term that includes both laboratory cultured blood and synthetic blood. Synthetic blood is still under research and is a completely artificial alternative that does not contain human cells. These modified molecules simulate the function of blood cells, delivering oxygen. It is mainly used in emergency situations or military medical care, as immediate oxygen delivery is required in these situations, but blood type matching is difficult.
Secondly, artificial blood has the potential to alleviate global blood shortages, especially in low - and middle-income countries. According to the World Health Organization, approximately 118.5 million people donate blood globally each year, with 40% coming from high-income countries, which account for only 16% of the global population. Recent studies have also shown that nearly 2000 units of blood are needed per 100000 people to meet global medical needs, but severe blood shortages still exist, especially in sub Saharan Africa, South Asia, and Oceania. In areas with extremely low blood supply, the bleeding mortality rate is significantly higher than in affluent countries.
On the other hand, laboratory cultured blood is produced by culturing human red blood cells in a controlled environment outside the body. Cedric Gwatt, a professor of transfusion medicine at the University of Cambridge in the UK, said that laboratory cultured blood cells, once available, could improve the treatment effectiveness of certain diseases. For example, compared to leukemia patients, laboratory cultured platelets may have better hemostatic effects in trauma patients. Leukemia patients receive platelets to prevent bleeding, not to stop it.
At present, the blood process cultivated in the laboratory begins with stem cells. Stem cells are a special type of cell that can develop into different types of cells in the body. These cells include red blood cells, platelets, and even skin cells, depending on the source and stimulation method of the stem cells. Scientists use a special type of cell called hematopoietic stem cells, which can produce all types of blood cells, including white blood cells, red blood cells, and platelets. They exist in the bone marrow or blood of donors. After a few weeks, stem cells gradually transform into mature red blood cells and perform the same functions as natural red blood cells.
However, laboratory cultivation or synthesis of blood products is currently only in the research and development stage. In 2022, a clinical trial in the UK marked a milestone by introducing laboratory grown red blood cells into human volunteers to evaluate their safety standards and lifespan. The product requires further testing before obtaining medical approval for commercial use. In addition, the current cost of cultivating blood in production laboratories is much higher than using donated blood. In 2013, the Defense Advanced Research Projects Agency (DARPA), a US government agency, reported that the cost of chemical materials required to produce a unit of laboratory cultured blood exceeded $90000.
And there are still some obstacles on the road to commercial production of blood. This includes how to increase production to meet clinical needs while ensuring the safety and functionality of laboratory cultured or synthesized blood products. In addition, regulatory agencies such as the US Food and Drug Administration and the European Medicines Agency are still determining whether laboratory cultured blood should be classified as cell therapy or drug, which will determine how it will be regulated.
Overall, the core value of artificial blood lies in compensating for the limitations of natural blood, enhancing medical accessibility and safety. Although it has not completely replaced natural blood, it has shown potential in emergency care, special scenarios, and scientific research fields, and future technological advancements may promote its wider application.

YTN TV of South Korea reported on Tuesday (December 16) that the South Korean court plans to make a ruling on the charges of former President Yoon Suk Yeol for obstructing justice on January 16, 2026.
YTN TV of South Korea reported on Tuesday (December 16) tha…
On December 7, a new round of intense military conflict bro…
Recently, US media disclosed that the Pentagon is planning …
From three launch failures and a brush with bankruptcy to n…
Recently, a major piece of news has emerged in the US polit…
Against the backdrop of the Federal Reserve's third rate cu…